FDA approves Narcan for over-the-counter sale to "combat the overdose crisis"
The U.S. recorded 101,750 overdose deaths from 2021 to 2022, mostly caused by synthetic opioids such as fentanyl.
VCU Capital News Service / Flickr.
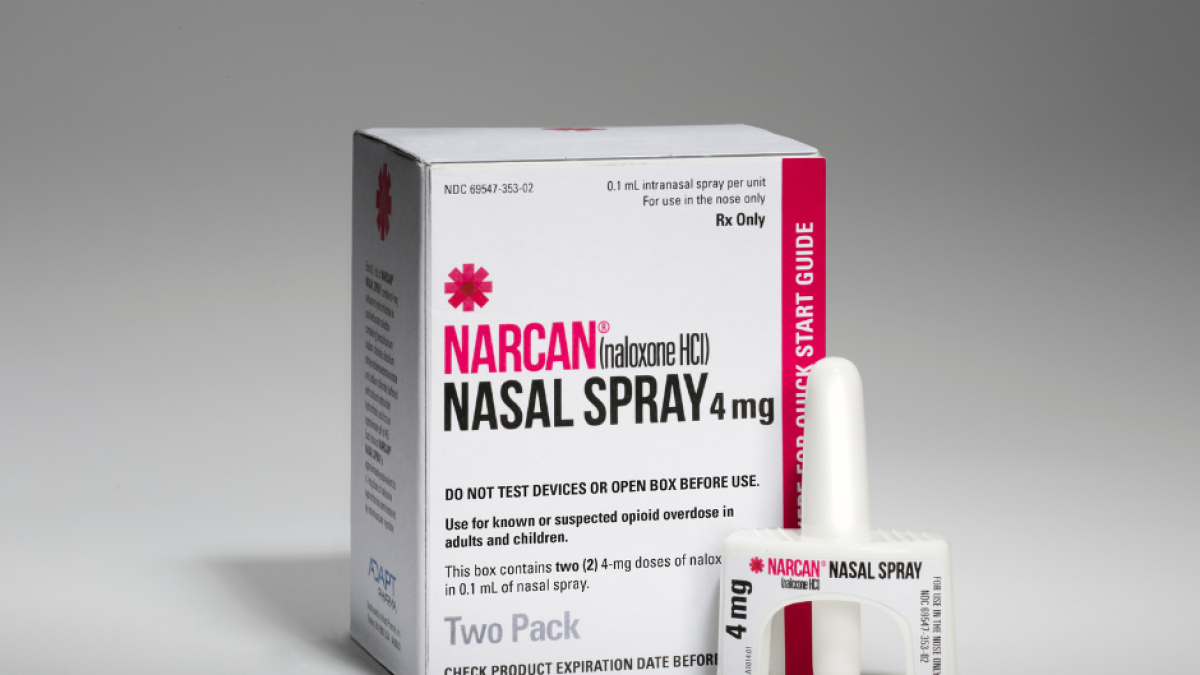
The growing crisis of excessive drug use in the country led the Food and Drug Administration (FDA) on Wednesday to approve Narcan, an opioid overdose reversal medication, for over-the-counter sale without a prescription.
In a statement, the FDA reported that the nasal spray is the first naloxone product approved for over-the-counter use. This drug reverses the effects of an overdose and is the standard treatment against the life-threatening effects of opioids:
Emergent BioSolutions, the company that produces Narcan, said the drug will be available in pharmacies, authorized stores and even online by the end of the summer.
Some doses will only be available by prescription
The drug was first approved by the FDA in 2015, but it needed to be prescribed by a specialist. As for its price, two doses usually cost around $50.
Currently, despite its over-the-counter sale, some formulations and high doses of the drug will still be sold by prescription only:
Drug crisis
The country recorded 101,750 overdose deaths from Oct. 1, 2021 to Sept. 30, 2022. Many of these were caused by synthetic opioids such as fentanyl. As previously reported by Voz Media, overdose deaths among children and adolescents soared 113% from 2020 to 2021, registering the highest rate in decades.
Patrizia Cavazzoni, director of the FDA's Center for Drug Evaluation and Research said the approval will help "combat the overdose crisis."